Clinical Trials
Clinical trials are a type of research involving a group of volunteers that studies new tests and treatments (e.g. vaccines, drugs, dietary supplements, medical devices, medical procedures and behavioral intervention) to evaluate their safety and effects on human health outcomes. When proved to be effective and safe, these treatments may become tomorrow’s standard of care.
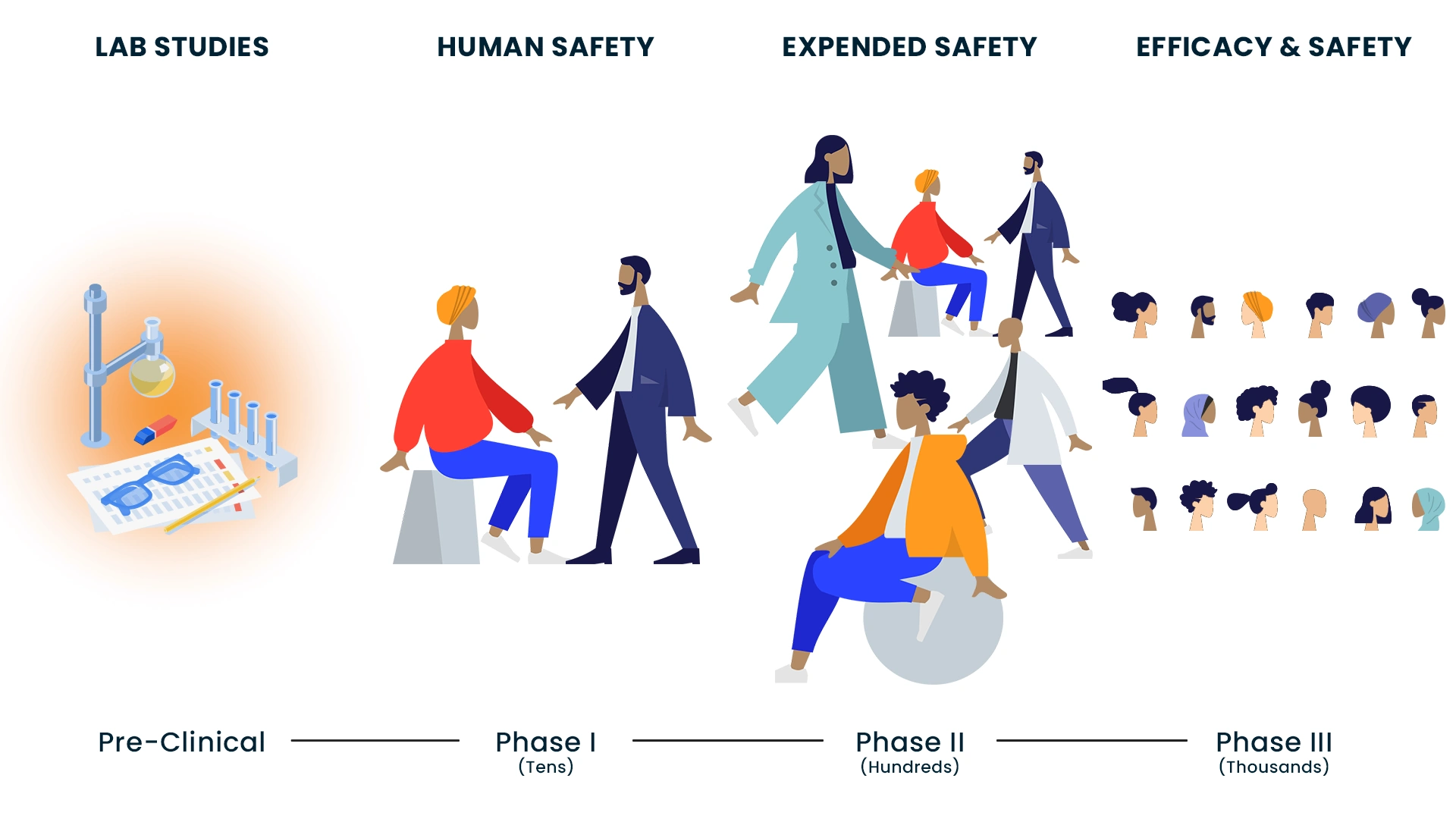
Clinical trials are part of a long, meticulous process, which may take many years. Research scientists may study a new treatment in a lab, in animals and also in people. This is done in three to four steps, or what is known as phases of clinical trials. Your doctor may offer you a clinical trial as a treatment option.
Clinical trials are carefully designed, reviewed, and need to be approved by the Ethics Committee (e.g. Medical Research and Ethics Committee/ PHKL Research and Ethics Committee) and Regulatory Authorities (e.g. National Pharmaceutical Regulatory Agency/ FDA) before they can start.
- Types of Clinical Trials
- Clinical Trials Phases
- Clinical Research Centre @ Pantai Hospital Kuala Lumpur
- Frequently Asked Questions

Access to medical care by
a multidisciplinary team
Access to potentially
life-saving treatments
Contributing to
scientific research

List of Trials
Explore various information available to assist you in discovering a clinical trial that aligns with your interests and potential participation.
Learn More
Access to medical care by
a multidisciplinary team

Access to potentially
life-saving treatments

Contributing to
scientific research





.png?sfvrsn=71934c60_8)




.png?sfvrsn=1370dfbd_9)

